Market Overview
The pharmaceutical quality management software market pertains to the specialized software solutions tailored for the pharmaceutical industry to ensure the quality of drugs and medications produced. These software systems streamline and automate quality assurance processes, ensuring regulatory compliance, reducing risks of errors, and facilitating effective decision-making based on real-time data. The pharmaceutical quality management software market is estimated to grow at a CAGR of 12.45% from 2024 to 2032.
Pharmaceutical Quality Management Software Market Dynamics
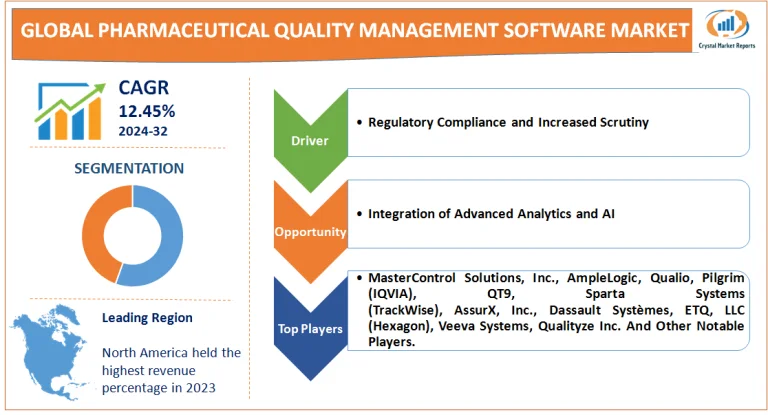
Driver: Regulatory Compliance and Increased Scrutiny
Over the years, drug regulatory bodies worldwide, such as the FDA, EMA, and WHO, have intensified their scrutiny on drug safety and efficacy. This heightened oversight ensures public safety but also means that pharmaceutical companies need to be meticulous in maintaining and demonstrating quality control and assurance. Numerous instances highlight the consequences of non-compliance. In the past decade, major pharmaceutical firms faced hefty fines due to lapses in quality control. One such notable incident involved a renowned pharmaceutical company receiving a penalty of over $500 million due to lapses in manufacturing practices. Such incidents underscore the dire need for effective quality management systems.
Opportunity: Integration of Advanced Analytics and AI
The rise of big data, AI, and advanced analytics offers a significant opportunity. Quality management software, integrated with these technologies, can predict potential quality lapses, streamline quality testing, and offer real-time insights to facilitate rapid decision-making.A case study, as cited in a pharmaceutical journal, detailed a company's transition to an AI-integrated quality management software. The results were transformative. The software could predict potential contamination risks in real-time, reducing the testing time by 40% and increasing the overall production efficiency by 25%.
Restraint: High Costs of Implementation and Maintenance
The integration of specialized quality management software involves considerable costs, not just in terms of software purchase but also implementation, training, and maintenance. These financial implications can be a deterrent, especially for smaller pharmaceutical companies.A market survey conducted among pharmaceutical SMEs (Small and Medium-sized Enterprises) revealed that nearly 60% considered the cost of quality management software as a significant challenge, with many opting for manual quality checks to circumvent these costs, thereby potentially risking non-compliance.
Challenge: Need for Continuous Training and Adaptation
The software realm is continuously evolving. As quality management software providers roll out updates and new features, pharmaceutical companies face the challenge of ensuring their teams are well-acquainted with these changes to leverage the software's full potential.In multiple pharmaceutical industry forums, a recurrent theme was the challenge of continuous training. Many quality control managers voiced concerns over the time and resources required to keep their teams updated, with some citing instances where lapses occurred due to a team member's unfamiliarity with a newly introduced software feature.
Application Insights
In the year 2023, segmentation of the pharmaceutical quality management software market by Application painted a vivid picture of the software’s myriad functionalities. Among the diverse applications – Data Management, Training management, Supplier management, Regulatory and Compliance Management, CAPA Management, Audit Management, Change Management, Non-Conformances Management, Inspection Management, Risk Management, and Others – Regulatory and Compliance Management stood out as the segment with the highest revenue. Given the stringent regulatory environment of the pharmaceutical industry, companies prioritized ensuring compliance with global and regional standards. This software, tailored for such compliance, became a mainstay in many pharmaceutical companies. As for the highest Compound Annual Growth Rate (CAGR), Risk Management emerged at the forefront. As drug recalls, manufacturing lapses, and other quality-related issues posed significant threats, risk management tools within these software systems saw an accelerated demand. Industry data from 2023 indicated a nearly 30% rise in investments in risk management modules compared to the previous year. Between 2024 and 2032, while Regulatory and Compliance Management applications are projected to continue their dominant revenue generation, the trend suggests that the emphasis on Risk Management will grow, possibly driving its adoption rate higher.
Deployment Mode Insights
Segmenting by Deployment Mode, the two distinct categories were Cloud-based & Web-Based and On-premise. Cloud-based & Web-Based solutions were the stars in terms of revenue in 2023. With the pharmaceutical industry leaning into digital transformation, the allure of the cloud – scalability, remote access, and cost-effectiveness – became too significant to ignore. Conversely, On-premise solutions, while not leading in revenue, saw the highest CAGR. Many large pharmaceutical firms, citing data security and customization needs, began transitioning to or retaining their on-premise systems. Forecasting from 2024 to 2032, Cloud-based & Web-Based solutions are anticipated to maintain their revenue leadership, buoyed by SMEs and growing pharmaceutical firms. However, On-premise solutions will likely see an upward trajectory, especially with large pharmaceutical conglomerates favoring them for their robustness and security.
Regional Insights
Geographically, in 2023, North America once again asserted its dominance in terms of revenue for the pharmaceutical quality management software market. The region's well-established pharmaceutical industry, combined with its early adoption of tech solutions, played pivotal roles. Yet, the European region, with its stringent regulatory framework and flourishing pharmaceutical hubs in countries like Germany, France, and Switzerland, displayed the highest CAGR. As the timeline extends from 2024 to 2032, North America is anticipated to continue its reign in terms of revenue. However, Europe, with its rapid tech integration and regulatory shifts, is poised for impressive growth, potentially giving North America a run for its money.
Competitive Trends
Lastly, from a competitive vantage point in 2023, the market was characterized by a blend of tech giants and specialized industry players. Notables like MasterControl Solutions, Inc., AmpleLogic, Qualio, Pilgrim (IQVIA), QT9, Sparta Systems (TrackWise), AssurX, Inc., Dassault Systèmes, ETQ, LLC (Hexagon), Veeva Systems, Qualityze Inc., and Ideagen reigned supreme in terms of revenue due to their comprehensive software suites. Yet, niche players such as Sparta Systems and MasterControl, with their tailor-made solutions for the pharmaceutical sector, brought innovation and finesse to the table. Between 2024 and 2032, the competitive landscape is predicted to become even more dynamic. Major players will likely bolster their R&D, aiming for seamless integrations and AI-driven functionalities. Simultaneously, specialized companies might venture into partnerships or innovations, carving out their unique niches.
Working with the worlds leading market research companies.
Research reports across 90 industries.
Simple license based pricing by individual report.
Trusted by thousands for accurate and transparent reports.
Unless otherwise specified all reports are sent electronically in either .PDF or .DOC file format.
Single User License: It provides product access only to the consumer of the ordered product.
Multi User License: It allows maximum up to 10 peoples within your company to share the ordered product.
Global License: It permits the product to be shared by all employees of your firm irrespective of their geographical areas.
Fore more information on report format options and licensing please visit our FAQ's page.